Early detection in cancer is crucial for improving patient outcomes, reducing treatment burden, and lowering healthcare costs for one of our largest global health concerns. Biomarker sequencing, enabled by advancements in genomics and sequencing technologies, has revolutionised cancer diagnostics by identifying specific molecules indicative of cancer development and progression. Multi-cancer early-detection liquid biopsies offer a promising approach for non-invasive and comprehensive cancer screening. However, successful implementation and adoption of these technologies require careful consideration of ethical, logistical, and regulatory challenges. To navigate these challenges, manufacturers must segment target populations to avoid overdiagnosis, facilitate the implementation of relevant technologies to allow the scaling of tests, as well as foster relationships with KOLs to ensure they are incorporated into clinical guidelines respectively.
Cancer poses significant challenges to our health, well-being, and the global healthcare community. As the second leading cause of global mortality, after cardiovascular diseases [1], it demands substantial attention and resources. Efforts to comprehend its complexities and develop effective treatment strategies persist, with the oncology pipeline representing more than a third of all speciality drugs in development in the US [2]. Our calculations have revealed that the direct medical costs incurred by the economy due to the treatment of the five most prevalent cancers exceed an astounding $520 billion annually.
An alternative approach to lowering the burden of cancer, aside from improving treatments, is through cancer screening and early diagnosis. When cancer care is delayed there is a lower chance of survival, greater problems associated with treatment and higher costs of care. In fact, the 5-year survival rate for the three most common cancers (breast, bowel, lung) is more than 6 times higher when the disease is diagnosed at the earliest stage compared to late-stage diagnosis [3]. Additionally, medical expenses have been shown to escalate significantly with each stage of cancer, as depicted in Table 1 [4]. The data highlights that, on average, a savings of $25,000 per patient can be achieved in the first 12 months by diagnosing the disease just one stage earlier.
| Tumour Stage | Average Costs per Patient (12 months after diagnosis) (USD) |
| 0 | $60,000 |
| I/II | $82,000 |
| III | $130,000 |
| IV | $135,000 |
Table 1: Estimated average 12-month costs following diagnosis for each cancer stage [4]
While tissue biopsy serves as a reliable tool for detecting and assessing cancer, its accessibility can be invasive and challenging, particularly in cases of metastatic diseases like late-stage lung cancer. Furthermore, tissue biopsy is impractical for early cancer screening when tumours have not yet formed. Although existing screening methods, such as mammograms for breast cancer, Pap tests for cervical cancer, and low dose computed tomography for colorectal cancer, have demonstrated effectiveness for specific types, they are limited in terms of sensitivity, specificity, and applicability to a single cancer type. In the United States, guidelines only recommend routine screening for four cancer types (breast, cervical, colorectal, and lung) [5], leaving approximately 70% of cancer deaths without standardised screening protocols [6]. Evidently, there is a pressing need for a comprehensive, cost-effective, and widely applicable cancer screening protocol to meet the demands of large-scale screening efforts.
Biomarkers have the potential to assume a critical role in cancer diagnostics as they encompass unique molecules, including genes, proteins, or other substances, that act as measurable indicators essential for detecting, diagnosing, and monitoring cancer within the body. DNA sequencing methods serve as a powerful tool for identifying cancer biomarkers, as they enable the precise determination of the sequence of nucleotide bases within a sample, allowing for the detection of genetic alteration and patterns of gene expression that are indicative of cancer development and progression. However, traditional methods of biomarker identification and DNA sequencing, such as Sanger sequencing, often involved studying one or a few genes or proteins at a time, which was laborious, time-consuming, and expensive. For instance, the sequencing of the entire human genome, encompassing the complete set of genetic information in humans, was achieved through the Sanger sequencing method. This monumental endeavour demanded nearly 15 years of intensive collaborations among numerous laboratories worldwide and cost approximately 100 million USD [7]. This bottleneck was preventing us from using these biomarkers to their full potential.
However, advancements in genomics and sequencing technologies over the last two decades, spearheaded by Illumina Technologies, Pacific Biosciences and Thermo-Fisher Scientific, to name a few, have successfully unlocked this opportunity. The development of second and third-generation sequencing technologies, often termed next-generation sequencing (NGS), offer a high-throughput and cost-effective approach to reading large amounts of DNA or RNA. The major market leader Illumina, which controls about 80% of the global DNA sequencing market, unveiled their NovaSeq X series late last year, which will reduce the sequencing cost to 200 USD per human genome while having the power to sequence more than 20,000 genomes per year [8]. This dramatic cost reduction and expanding capacity have significantly increased the accessibility of the technology, exemplified by the recent collaboration between Thermo Fisher Scientific and Pfizer to extend its reach to over 30 countries lacking affordable access to advanced genomic testing [9].
By leveraging the power of NGS, researchers have made significant strides in identifying novel cancer biomarkers across various cancer types. These biomarkers can be used for early detection, stratification of patients into appropriate treatment groups, and monitoring of treatment response, ultimately leading to more precise and personalised cancer care. The industry is now poised for rapid growth because of recent advances in the field, including more sophisticated diagnostic technologies and a great understanding of disease heterogeneity.
With these NGS technologies in their arsenal, researchers and start-ups have now cast their eyes on liquid biopsies – a revolutionary blood test designed to detect circulating cancer DNA. As cells die, DNA fragments are expelled and circulate within the bloodstream. The rapid proliferation of tumour cells results in heightened cell death, leading to an excess of cell-free DNA being released into the blood, commonly referred to as circulating tumour DNA (ctDNA). The tumour-specific mutations in the ctDNA sequence can act as a new type of cancer biomarker and help to identify cancer patients from a group of healthy individuals, as described in Figure 1. Several companies are leveraging these markers to develop a single liquid biopsy test that can diagnose several cancers. These innovative screening tests, referred to as multi-cancer early detection (MCED) tests, employ NGS and artificial intelligence to identify and analyse ctDNA patterns. By doing so, they can accurately identify and locate cancerous tumours. In comparison to the conventional approach of cancer diagnosis using tissue biopsies, these MCED liquid biopsy tests offer earlier detection, scalability, minimally invasive procedures, and hold great potential for detecting rarer forms of cancer.
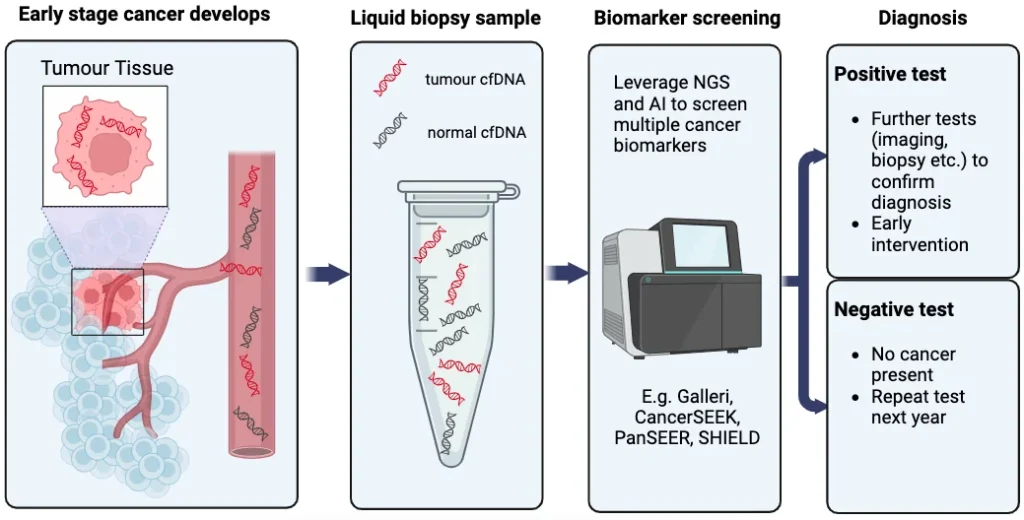
Figure 1: A basic overview of the MCED test journey. [Illustration made by S&S]
There are several early detection tests harnessing these concepts that are currently in development, some of which are presented in Figure 2. The competitive landscape is rapidly evolving, with numerous companies and start-ups actively pursuing advancements in the field. A few key players have emerged, each striving to develop their own proprietary technologies and methodologies for comprehensive cancer screening.
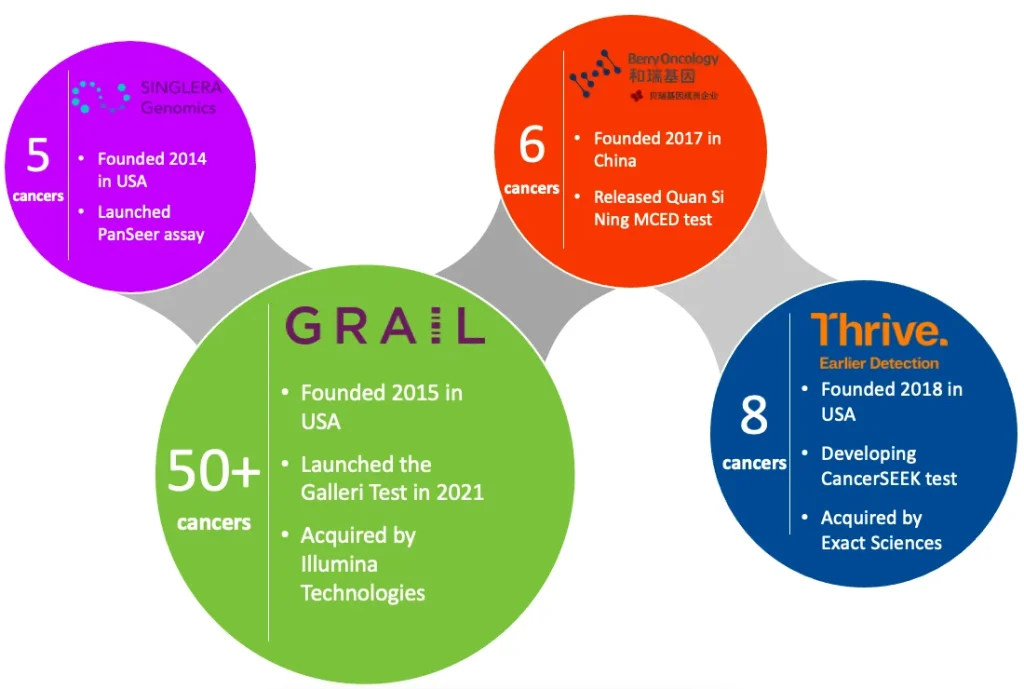
Figure 2: A non-exhaustive mapping of the current MCED market landscape.
The Galleri test developed by GRAIL stands out as particularly promising. This innovative test harnesses the power of NGS and machine-learning algorithms to analyse indicative methylation patterns observed in ctDNA. The test boasts the capability to identify a distinct signal that is shared by more than 50 different types of cancer, and their latest findings provide compelling support for this performance claim [10]. To enhance the adoption and reach of the product, GRAIL is undertaking multiple strategic initiatives, including a collaborative effort with the U.S. Department of Veterans Affairs to screen 10,000 veterans [11]. Furthermore, the company is currently conducting a large-scale study with the National Health Service involving approximately 140,000 participants in the UK [12]. This ambitious endeavour is projected to conclude by early 2026, aiming to provide the necessary evidence required to prove its efficacy for early detection. Although Galleri is yet to obtain Food and Drug Administration (FDA) approval, the test is available to older patients in the U.S. with a higher cancer risk under a Clinical Laboratory Improvement Amendments (CLIA) waiver [13].
Acknowledging the immense potential of this technology, GRAIL was acquired in 2021 by Illumina Technologies, the aforementioned front-runner in NGS technologies, for an astounding $7.1 billion [14]. However, to foster competition and lower market entry barriers, regulators in the United States and Europe have mandated Illumina to divest GRAIL in early April of 2023 [15]. This action by the Federal Trade Commission underscores their recognition of the technology as a critical solution, indicating ample room for further growth and the entry of other players into the market.
In 2019, Thrive entered the field of multi-cancer early detection with their CancerSEEK technology backed by $110 million in Series A funding, marking the largest investment ever for a John Hopkins-licensed technology [16]. Their MCED liquid biopsy test combines genetic mutations in cfDNA with the analysis of nine proteins, enabling screening for eight prevalent cancer types that account for over 60% of cancer-related deaths in the U.S. Notably, this includes four cancer types for which there are currently no available screening tests. Conducting a comprehensive study involving over 1,000 patients, Thrive achieved an impressive average detection rate of 70%, catching 98% of ovarian cancers [16]. Despite the scope of CancerSEEKs screening range being narrower than that of Galleri, Thrive was still acquired by Exact Sciences (a molecular diagnostics multinational) for up to $2.15 billion just 2 years after its establishment [17].
Although these tests are not meant to replace existing screening methods (mammograms, Pap tests, colonoscopy, PSA blood tests, etc.), they can serve as valuable complements to current screening strategies and aid in the detection of other types of cancers lacking established screening protocols. But of course, much work is needed, and with experience and larger samples, these assays will improve. With sequencing consistently becoming cheaper and more accessible, it is only a matter of time before this technology is made viable. This leads us to the next considerable challenge – how will this technology be implemented?
Companies with new early cancer detection technologies need to be vigilant and address several key aspects to ensure successful implementation and adoption:

Firstly, the regulatory landscape plays a vital role in the implementation of MCED tests. Striking a balance between facilitating innovation and ensuring rigorous oversight is essential. Regulatory authorities need to establish clear guidelines and standards for the use of these tests, positioning the technology to promote public trust whilst fully leveraging its potential. This undertaking is far from simple, as exemplified by the utilisation of prostate-specific antigen (PSA) in cancer screening, where the ongoing controversy regarding the trade-off between screening benefits and the risks of overdiagnosis and overtreatment has resulted in divergent protocols between the US and UK [19-20]. It is evident that regulatory guidelines can differ across countries, further complicating the journey towards achieving global market access.

Furthermore, the integration of multi-cancer early detection tests into the existing healthcare system presents logistical and economic challenges. These tests often involve complex technologies, such as the discussed NGS technologies and advanced data analytics, requiring specialised infrastructure and expertise. Implementing these tests on a large scale demands substantial investments in laboratory capabilities, efficient workflows, and healthcare professional training.

To drive market adoption, active engagement with stakeholders is essential. Key opinion leaders (KOLs) play a crucial role in disseminating best practices, establishing guidelines, and advocating for the adoption of MCED tests. Collaborating with healthcare professionals enables education and heightened awareness about the numerous benefits of early cancer detection. Moreover, establishing favourable reimbursement policies through collaboration with insurers and policymakers facilitates the seamless integration of these tests into healthcare systems. By actively engaging with stakeholders, manufacturers can accelerate the widespread adoption of MCED tests and enhance the impact of early cancer detection efforts.
Lastly, the successful implementation of this ground-breaking technology necessitates a comprehensive strategic analysis to identify the target populations. Certain individuals without symptoms may harbour a non-life-threatening cancerous tumour that has minimal impact on their quality of life. It raises ethical considerations about whether it is justifiable to impose upon them the stress of a diagnosis and the potential side effects of treatments. Moreover, the issue of false-positive results should be considered. Galleri, for instance, currently boasts a sensitivity of 99%, implying that 1 in 200 individuals may receive a false-positive result [18]. While these statistics are likely to improve as the product evolves, it begs the question of whether subjecting the general population to the emotional burden of a false-positive outcome is sensible. Alternatively, it may be more prudent to categorise populations into risk groups based on factors such as age, genetics or specific exposures.
After all this extensive research into the technology and the validation of its efficacy, companies now need to begin considering their implementation strategies to ensure optimal market adoption. With a wealth of experience, the Sector & Segment team excels in researching and quantifying the specific needs, attitudes, and preferences of key stakeholders. By leveraging our expertise, we can help companies in the early cancer detection space pinpoint the differentiating factors that will drive success in the market. Specifically, our experts can provide support through: