

Margarita Svarceva

Mainstream treatment of chronic wounds relies on passive bandage and gauzes which do not allow the reconfiguration of the structure and function of damaged skin, resulting in stalled healing, potentially even amputation and death. Advanced dressings offer more than just protection for chronic wounds – they can promote healing, speed up tissue regeneration and can improve care outcomes. Innovation in the wound care space is largely shaped by the dynamics between reimbursement policies, health economics and clinical evidence. With lack of a standard treatment protocol in place, any innovation that can offer clear value proposition can stand a chance for market adoption. In this piece we share the insights from our interviews with industry experts and leading academics on the future of chronic wound care with a look at what’s new, what’s emerging, and what’s next in the chronic wound dressings space.
In chronic wounds, the natural healing process can be stalled by damage or loss of the extracellular matrix of the dermis, that holds tissues in place as a scaffolding and signals for new cell formation. Wound closure by skin grafting can offer integration of living tissues with wound bed to help restart the healing and cell regeneration. In this respect, autografts are considered as the gold-standard in skin replacement in wound care.[1] By transplanting a piece of healthy skin from patient’s own body, it offers a reduced infection risk, low risk of immune reaction and promotes revascularisation of the wound with patient’s own living cells. However, autograft harvesting may not always be feasible when the wound is extensive, or if the patient cannot sustain a second wound site. Consequently, a variety of skin substitutes that can replace the extracellular matrix function of the skin have been launched.
Allografts (from cadaveric human skin) or xenografts (from animals) emerged as viable alternatives to autografting. MiMedx’s EpiFix is an allograft example that has been on the market since 2012 that has clinically shown statistically significant wound closure results in randomised controlled trials.[2] As human tissue products, allografts are exempt from FDA approval whereas xenografts have to receive 510(k) “medical device” clearance for market access. However, they are expensive to source, and expensive to store.
A viable skin substitute option is the fish-skin grafts of Kerecis (Coloplast -since 2023) which are sourced from Atlantic cod and have been on the market since their FDA approval in 2013. Over the years clinical evidence expanded their use from burns to chronic wounds. There are currently ongoing clinical trials with Venous Leg Ulcer patients and recent publications have looked at intact fish skin grafts’ effectiveness in treating deep Diabetic Foot Ulcers.[3][4][5] Evidence so far has even showed Kerecis performed significantly faster in full thickness wound closure when compared to EpiFix.[6]

Another promising example is the porcine-placenta derived InnovaMatrix by Convatec – which received its FDA approval as a medical device designation in 2022.[7] Porcine xenografts, just like fish-skin xenografts, are histologically similar to the human dermis. Convatec is currently running randomised controlled trials looking at its efficacy in wound closure with Diabetic Foot Ulcers and Venous Leg Ulcers.[8][9][10]
When compared to allografts, animal-derived xenografts are easier to procure making them advantageous for continuous, as well as mass use.[11] However, a key challenge they face is the possibility of rejection by the patient – either due to having immune reaction after use, or outright objection to use on religious grounds (i.e. porcine).
Some Chronic wounds require periodic debridement and changing of dressings to manage exudate or to prevent infections. Unfortunately, frequent dressing changes or debridement can be painful and can also delay epithelisation of the wound site by removing newly formed skin cells while targeting removal of biofilm. Dressings that can both fight off infections and be absorbed in the wound site can eliminate disruptions in the healing process.

To this end, Renovoderm’s bioabsorbable 3D electrospun Phoenix Matrix’s currently in trial, is looking at how it alters the wound microbiome in Diabetic Foot Ulcers.[12] Having received FDA approval in 2018 for burn wounds, it’s steadily building evidence for its use with chronic wounds. Phoenix wound matrix can dissolve in the wound site over the span of 7-14 days, and comes with the benefit of lowering pH in the wound site to fight off infections.
Effectiveness of bioabsorbable dressings was also noted in the latest update to the NICE Guidelines (UK) with respect to application of polyurethane film dressings.[13]PolyNovo’s NovoSorb BTM is a biodegradable polyurethane dressing on the market that fits the NICE recommendations. It has recently received a product extension in 2024 with NovoSorb MTX which is the same as BTM without the attached sealing membrane, allowing for a seamless integration to facilitate wound closure.[14]
Chronic wounds – particularly Diabetic Foot Ulcers present as heterogenous conditions. The extent, severity, progression, co-morbidities and underlying causes can differ from patient to patient. Further, the wound site microbiota, surface area and ulcer depth can determine the response to treatment. Delivering bioactives directly to the wound site with appropriate dressings can be a challenge, but the use of electrospun ECMs as a drug delivery system present a viable solution both in terms of cost and in terms of practicality.
Electrospinning has been around for almost a century. However, the technology has improved so much that ultra-thin nanofibers can now be produced through this method. These nanofibers can be shaped and layered in such a way that can mimic the natural porous character of human dermis which allows for moisture management at the wound bed. The aforementioned Phoenix Matrix and MedSkin’s Matriderm (FDA cleared in 2021) are two examples of dressings on the market that leverage the electrospinning technology.
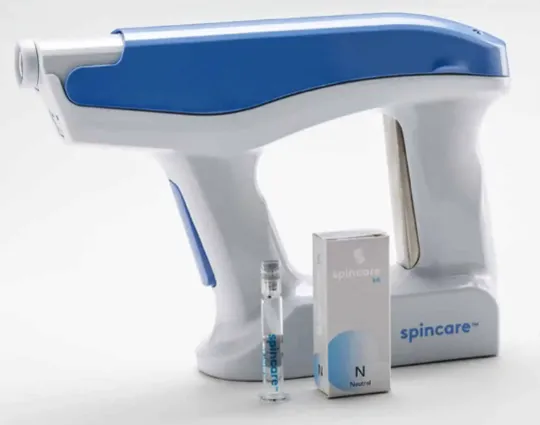
Nanomedic’s Spincare integrated the electrospinning method -which typically takes place in a lab environment- into a portable device. This handheld device is currently only available in the European Union (having received CE mark) and is in the submission stage for FDA approval to launch in the US market. Spincare produces dressings in real-time that take account of the wound landscape and shoots over electrospun nanofibres directly on to patient’s skin. The syringe with the polymer solution can be enriched with antibacterials, antibiotics, collagen or cannabis to deliver bioactives directly to the wound bed while allowing moisture control, and they can allow for a slow release of these bioactives making it a strong contender for an effective drug delivery platform. Although the clinical evidence for their application in chronic wounds is limited, this could change in the near future.[15]A customised electrospun mixture with the agility of a portable crafting of a personalised dressing can be effective in outpatient and community settings.

Looking beyond electrospun ECMs, other alternatives or patented technologies are also emerging to facilitate drug delivery to the wound site. Convatec’s purchase of 30 Technology with its patented antimicrobial nitric oxide platform in 2023 has allowed for Convatec’s launch of ConvaNiox: antimicrobial nitric oxide-generating dressing.[16] With a clinical trial planned to conclude later this year, ConvaNiox is planned for phased launch over late 2025-2026 and is a candidate to become the go-to product in the management of chronic wounds prone to infection.
Growth factors are proteins that regulate cell activity and play a key role in stimulating tissue regeneration. Their presence can regulate and regenerate cell activity in a wound site, however delivering them in a stable form has been a challenge. In the context of chronic wounds, growth factors have been approved for use in the form of FGF-2 in China and Japan, EGF in Cuba but there exist discrepancies in adoption across countries.

Rion Inc.’s Purified Exosome Product (PEP) is a human platelet powder that is rich in growth factors such as TGF-beta. It can be topically applied with saline over the wound bed and is currently undergoing Phase 2 clinical trials for their use with diabetic foot ulcers.[17] While they are not considered a “dressing” in the conventional use of the word – this powder can be used alongside hydrogels or fibrin sealants such as TISSEEL (Baxter) and can be covered with a secondary dressing. The application of PEP promises a “healing from within” approach, where it assists with cell and tissue regeneration. From a regulatory perspective, what sets the growth factor dressings apart is the higher threshold they have to follow in terms of their approval process: while bioactive dressings are considered as medical devices by the FDA, growth factors are evaluated as biologics and are bound to a higher approval threshold with their clinical evidence on par with therapeutics - that is to say randomised controlled trials evaluating efficacy.
When dealing with chronic wounds it is necessary to look beyond “wound closure” as an objective and take account of infection prevention. For some chronic wounds such as with Diabetic Foot Ulcers, wound beds are sites for opportunistic bacteria that can yield infections which are hard to manage. Antimicrobials are generally not recommended for use beyond 2 weeks to prevent antibiotic resistance. An emerging approach in this space is the use of probiotics.
When applied on wounds, probiotic bacteria can compete with harmful bacteria for available resources (i.e. oxygen) on the wound bed, killing off harmful bacteria in the process.[18] Innovative drug delivery mechanisms – such as electrospun ECMs – can include bioactives such as probiotics, to provide localised infection control where re-infection risk is high and long-term antibiotic use is not viable. A 2022 publication shows that the addition of friendly bacteria to the wound bed after debridement could help with wound healing.[19] [20] Research shows benefits of probiotic supplementation for gut microbiome health – extending the same logic to chronic wound management can offer an alternative treatment option. Clinical evidence in this area is growing, with research showing the role of topical probiotics on collagen production, capillary growth and in reducing biofilm in animal models.[21]
Despite academic research on their effectiveness, no probiotic wound dressings have been approved by the FDA so far. However, our industry interviews suggest that the awareness on probiotics is gaining momentum, so innovations in this space are expected within 3-5 years.
In conclusion, it is worth noting that there is a regulatory turn – particularly with the FDA – emphasising efficacy with wound dressings. Therefore, more evidence is likely going to emerge in wound care management that will distinguish innovations that can deliver on their promise. However, the likely fate of any innovation moving from bench to bedside will remain a matter of successful market access and adoption.
At Sector & Segment, we help you navigate the evolving wound care landscape by identifying high-value innovation opportunities and supporting your growth strategy. Whether you are an established player or a new entrant in the wound care space, we support you in transforming scientific promise into commercial success. Our team combines deep healthcare expertise with robust strategic capabilities to accelerate commercial success through:
We collect, use, analyse and share data such as statistical or marketplace data and provide information such as opinions and insights for general information purposes only. The content of this article is not intended to amount to advice of any kind. No reliance should be placed on any statements made in this article, whether for medical, health, legal purposes or otherwise. Nothing in this article is an offer to enter into a binding contract or a recommendation, endorsement, guarantee or warranty of any kind. The content of this article is aimed at industry institutional professionals and is intended to serve as a concise initial reference and not as a complete reference source. You must obtain medical, professional or specialist advice before taking, or refraining from, any action on the basis of the content in this article.
You acknowledge that the content of this article may contain inaccuracies or errors and we expressly exclude liability for any such inaccuracies, incompleteness or errors to the fullest extent permitted by law. Neither we nor any third parties provide any warranty or guarantee as to the accuracy, timeliness, performance, completeness, or suitability of the information herein for any particular purpose. Some information may contain links to other sites, resources, or opinions of third parties and are provided for your information only. We have no control over the contents of those sites or resources and are not responsible for the content. In no event shall we be responsible for any loss or damage of whatever kind (including negligence) arising out of or in connection with your use of or reliance on any content within this article. You agree that your use of this content is at your own risk. This does not affect claims in respect of death or personal injury caused by our negligence and or excludes or limits liability that cannot be limited under law.
Healthcare Categories
Med Tech
Therapeutic Areas
Wound Care


6 June 2025
Maria Franco Hernandez

18 March 2025
Steven Yap

4 November 2024
Maria Franco Hernandez
Strictly Necessary Cookies
These cookies allow the website to function properly, and it is not possible to use the website without them.
Statistical Cookies
These cookies allow statistical data to be collected about the use of our website.